Article
6 Engineering Supporting Hydrogen Development in the Foundation Industries
23/04/2025
Why all the buzz around hydrogen?
Hydrogen is a crucial piece of the UK’s puzzle to achieve Net Zero carbon emissions through a range of applications by 2050. It is an abundant and high gravimetric (MJ/kg) energy density fuel which, when combusted produces only water as a byproduct. Due to its high volatility and its very small molecule, there are several challenges relating to production, storage, transport and use which must be addressed to ensure delivery of energy targets are met safely.
Hydrogen has been used in the major accident hazard industries for several decades and is not a new substance in the engineering field. However, new applications are being developed at a rapid rate and innovation is occurring in areas where hydrogen is not a familiar substance.
In the UK (United Kingdom), the safe use of hydrogen is regulated through compliance with the Control of Major Accident Hazards (COMAH) regulations, Dangerous Substances and Explosive Atmospheres Regulations (DSEAR) and the Control of Substances Hazardous to Health (COSHH) regulations. However, the scale and profile of hydrogen use has been at a much lower volume and throughput than the applications being considered to support the transition to Net Zero. Because of this, most of the industry standards and guidance documentation are relevant to older practices than the latest applications may be using. And while the competent authorities and standards agencies are catching up, the most recent releases from the HSE and the trade relate to the direct use of hydrogen by the public such as hydrogen-powered vehicles and the introduction of a percentage of hydrogen blend into the national natural gas network.
6 Engineering are providing safety engineering support to several foundation industry partners on projects where hydrogen is being introduced as a direct-fired alternative fuel to natural gas in traditional practices including the production of iron and steel, cement and ceramics and the manufacture of products from foundation industry materials.
In the following sections, Aislin Brown, Safety Engineer at 6 Engineering explores some of the challenges and considerations that have occurred during our work with Lucideon as they adapt their new facility in Stone and their original heritage location in Stoke-on-Trent to support innovations in the ceramics industry through the installation of demonstration/semi-commercial scale hydrogen infrastructure that will inform and support the development of full-scale industrial applications.

Considerations when replacing natural gas for hydrogen
Hydrogen energy density, molecular mass, and the need for storage
Natural gas is supplied to industrial and domestic properties via a central pipeline network. Trials have demonstrated that 20% hydrogen can be blended with natural gas however the transfer to 100% hydrogen is still a way off. But supply via a network only covers some of the challenges associated with the use of hydrogen in direct firing. Whilst hydrogen has a higher gravimetric energy density than natural gas, it has a much lower volumetric density and burns at a much faster rate. This means a larger volume of hydrogen is needed to generate the same heat.
The natural gas distribution pipework for the UK operates at 7 bar on its medium pressure lines. This is reduced to required pressures at the site gas meter. Household boilers operate around 25mbar. By contrast, hydrogen bottles can be filled to 200 bar, increasing the fuel release from a comparable leak source and therefore increasing the scale of a potential incident while also increasing the complexity of pressure regulation equipment needed to balance supply with demand.
Many natural gas applications have designated hazardous area zones around the natural gas infrastructure; however, the classification and explosive atmosphere potential is often classified as ‘negligible extent.’ This is possible to achieve and maintain with suitable ventilation on low pressure pipework in part due to the range in concentration required to develop an explosive atmosphere (5-15% in air). Hydrogen applications are far more difficult to achieve this due to the wide range in concentration in air that hydrogen is flammable, (4-75% in air) leading to a wider zone and greater potential to generate an explosive atmosphere.
Hydrogen is incredibly light, much lighter than air, and will lift to the highest point possible from a release point. This can be beneficial in terms of the gas leaving a release area quickly, but can also result in accumulation, particularly in roof spaces with poor ventilation or circulation. Its buoyancy combined with the high flammability range for hydrogen can result in some very large hazardous area dimensions for high pressure releases such as storage areas.
Substance | Density (kg/m3) |
|---|---|
Hydrogen | 0.09 |
Methane | 0.716 |
Air | 1.29 |
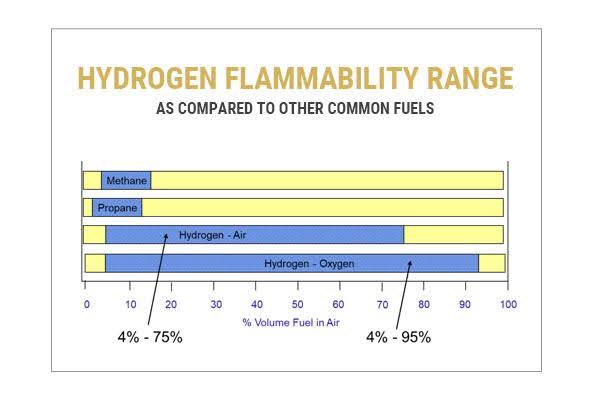
Hydrogen Flammability Range
This designation results in the introduction of ignition and leak controls to sites which may not previously have required such control measures. Existing installations looking to switch to hydrogen for their combustion processes will need to carry out detailed design studies to understand:
Pressure and volume potential from the supply grid and the demand of their combustion process.
The need for buffer storage and pressure regulation on site
The safe management of assets and infrastructure to demonstrate to the competent authority that risk of harm from an ignited loss of containment is as low as reasonably practicable
Appropriate hazard identification and risk assessment is undertaken throughout the design and installation process to ensure inherently safe design is achieved
Retrofitting hydrogen infrastructure to existing sites will require further assessments of risk such as occupied buildings risk assessment (OBRA) which could identify the need for emergency refuge and emergency planning, DSEAR risk assessments which will identify the risk of explosive atmosphere generation and support the categorisation of zones on site (Hazardous Area Classification) and the equipment that can and cannot be used in these zones.
Hydrogen materials, leak awareness, inspection, and maintenance
Hydrogen has an incredibly small molecule size and therefore a high tendency to leak. It can even pass through a steel pipe wall, given sufficient time and conditions to do so.
Substance | Van der Waal’s radius (nm) |
|---|---|
Hydrogen | 0.12 |
Methane | 0.68 |
Oxygen | 0.299 |
The choice of materials, number of fittings, operating pressures and maintenance procedures for the design and use of a hydrogen network are particularly important, and different in requirement to that of many other common gases.
As well as being extremely small and difficult to contain, hydrogen can affect the properties of other substances, particularly steel, through a process known as ‘embrittlement’ – where the resistance to cracking is lowered. This process takes time to occur and is not immediately visible, thus it requires suitable inspection and maintenance practices to control – especially for high pressure and high throughput situations.
Natural gas is treated with an odorant (80% tertiary butyl mercaptan, 20% dimethyl sulphide) which allows it to be detected by the human nose – that slightly sweet smell familiar to anyone who has walked past a gas working area or accidentally left the hob on. Due to its small molecule size and high buoyancy, there is not an odorant yet available which can be used to detect a hydrogen release at a representative size, and as such leaks must be detected through sound or pressure loss (infra-red cameras will not work). These are less likely to be noticed passive checks while walking through an area with hydrogen pipework, thus increasing the likelihood of accumulation of hydrogen gas into flammable quantities.
Hydrogen also has a very low minimum ignition energy of 0.017mJ (natural gas: 0.28) and has a positive Joule-Thomson coefficient; therefore, pressurised release of hydrogen can self-ignite. In fact, compressing hydrogen (for example, an impinged release) can lead to ignition. Hydrogen also has a wide detonability range of around 11-59% compared with that of natural gas which is 5.3-15.6%).
Managing hydrogen safely
Hydrogen is not a new fuel. Its properties in terms of molecular behaviour are well understood and its behaviour in release cases such as jets, tunnels, impinged flames, and other scenarios required for risk management are being expanded on daily, with practical research being undertaken by the HSE laboratories at Buxton, several universities and modelling companies; and that is just in the UK. There are several conglomerates across Europe and the world working together to ensure hydrogen is launched safely into industrial and public applications.
Petroleum is another flammable and hazardous substance, which you would not put in the hands of the untrained public based only off the safety data sheet. However, by addressing safety in the design stage for tankers, underground garage storage tanks and use of fuel pumps, many of the controls are in place without requiring specific training for every individual fuelling their car. Hydrogen can be similar: if focus is given to the correct stages of the design, whether this is the creation of a new vehicle fuelling system or the introduction of a hydrogen supply system to a site which requires direct heating, the risks can be managed, and suitable and proportional inherent safety systems applied.
Get in touch
6 Engineering are safety engineering professionals with a wealth of experience in the major hazard industries, research and pilot plant facilities, and implementation of safety systems. We are delighted to be supporting Lucideon with their installations in Stone and Stoke-on-Trent and welcome any enquiries for introducing or scaling up hydrogen use at your facility.
Visit our website www.6engineering.co.uk to get in touch; we look forward to hearing from you!
